
Clinker is the main constituent of cement. It is a nodular material produced in the pyroprocess stage of cement production and to the untrained eye, clinker looks like small grey rocks a few centimetres in diameter. However, when ground and combined with ~4% gypsum, clinker transforms into Ordinary Portland Cement – the key ingredient in concrete, the second most used material in the world, after water.
Clinker production is responsible for more than 90% of the emissions from cement production, which themselves are 8% of global CO2 emissions. This means that clinker production alone is responsible for around 7.2% of global CO2 emissions.
As we transition to a net-zero carbon world, clinker producers need to address their emissions which primarily come from two sources in the production process: – emissions from the fuel to reach high temperatures for the chemical reaction to occur (40% of clinker emissions) and natural emissions from the chemical reactions themselves (60% of clinker emissions).
Understanding the chemical reactions behind these sources of emissions is crucial for optimising cement production.
Clinker composition
We need a lot of lime (CaO), Silica (SiO2), Aluminium oxide (Al2O3) and Iron (Fe2O3) to create the primary constituents of clinker:
- Alite – which gives cement it’s early strength and helps cement set
- Belite – which contributes to the late strength of cement
The remaining molecules in clinker, C3A and, C4F (known as aluminates), are combinations of lime and aluminium oxide (alumina) and iron oxide.
There is also some trace free lime. These are Calcium Oxide (CaO) molecules that aren’t combined with any of these other compounds. Free lime is a crucial metric for cement plants and is closely controlled, as cements with high free lime content are weaker. This is because free lime hydration causes expansion in settled cement and therefore cracks that can weaken structural integrity.
Where do we find these materials?
The raw ingredients for clinker are:
- 75-85% limestone
- 10-15% silica
- 2-6% alumina
- 0.5-2% iron
Silica (SiO2), Aluminium oxide (Al2O3), and Iron (Fe2O3) are abundant in many sources, such as sand, shale, or clay. The most common way to obtain lime is to heat limestone, a sedimentary rock that is very easy to find.
Limestone or calcium carbonate has the chemical formula: CaCO3. At high heats of approximately 850 degrees celsius, limestone decomposes into lime and CO2 in a process called calcination:
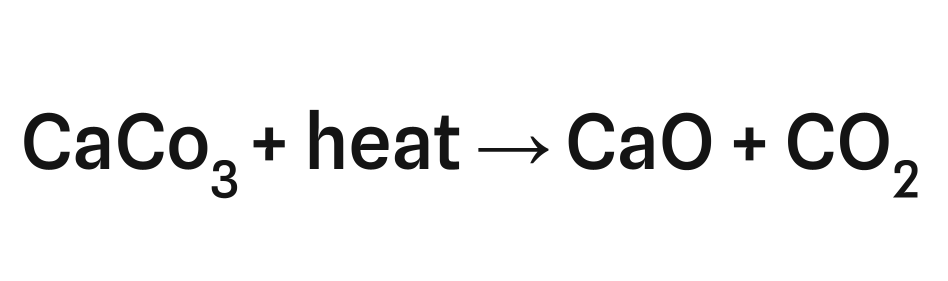
This single reaction is responsible for at least 4.3% of global CO2 emissions.
Once we have our base ingredients, we need to combine them to make the clinker molecules.
Any uncombined lime in this process becomes free lime. To minimise the uncombined free lime, the ratios of limestone, silica, aluminium, and iron must be carefully managed by Cement plants using the Lime Saturation Factor (LSF), which is essentially just a ratio of lime to the other oxides. This ratio fluctuates between 98% and 102% at most plants.
The production process
The decomposition of limestone into lime and CO2 takes place in the Preheater tower and Calciner. When the material reaches the rotary kiln, it is almost entirely calcinated (pure free lime). “Clinkerisation” reactions take place in the Kiln when temperatures reach 1450ºC. Tonnes of fossil fuels are burned constantly to reach these temperatures producing a lot of CO2. The mineral transformations in this process are summarised in the diagram below, where ‘carbonates’ are a combination of limestone and other carbon rocks (such as magnesite).
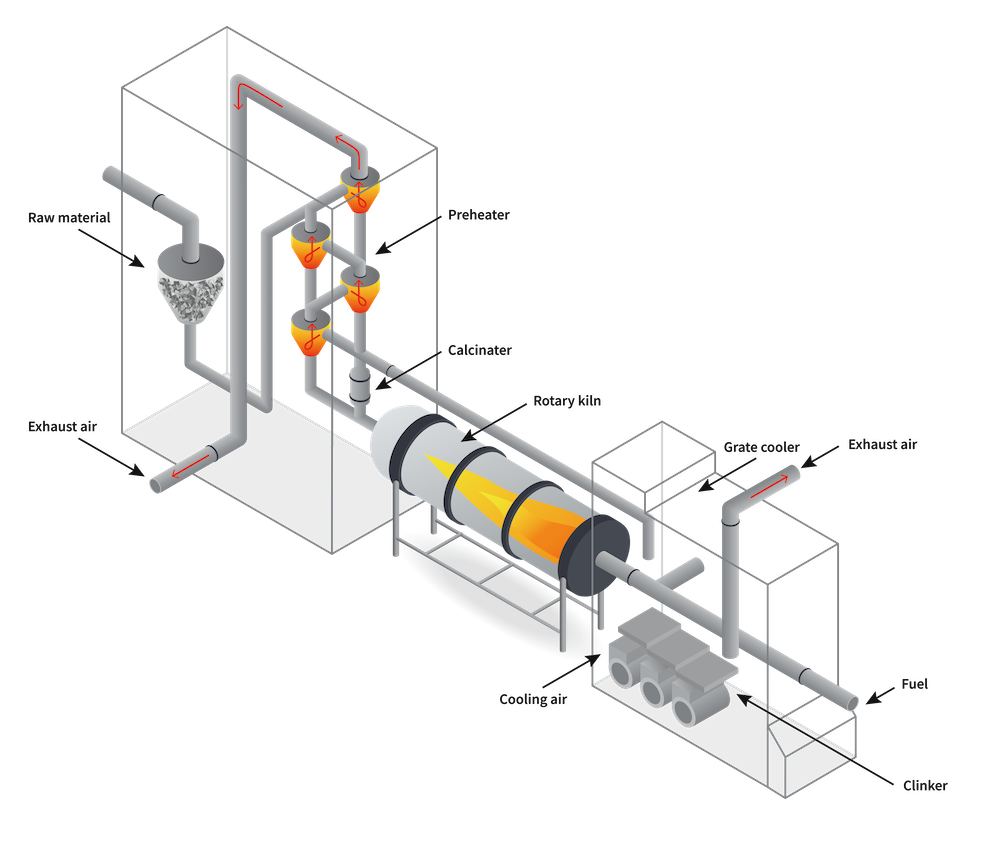
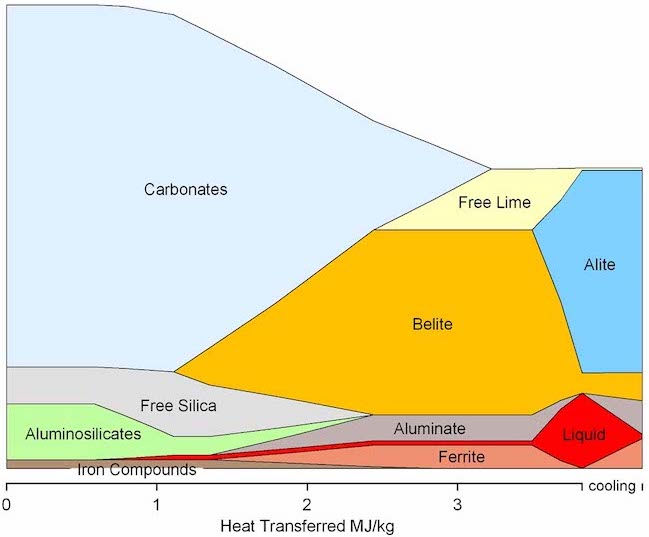
Left image:Diagram of a cement plant. The process moves from the left to right. Right image: Mineral transformation in clinker production. The bottom x-axis is in units of energy, but in the case of a cement plant, it is synonymous with a time axis.
Alite is very unstable between 1000ºC and 1400ºC, reverting back into belite. To prevent this reverse reaction, the clinker needs to be cooled rapidly to below 1000ºC, which takes place in the cooler.
The cooler quenches the heat from the clinker to “freeze” the alite (a bit like putting your soft-boiled eggs in an ice bath) and also recycles that heat back into the process, which is an effective way of reducing the amount of fuel that needs to be burnt.
As the clinker cools, it coagulates into little balls; this process is called nodulization. The plant samples these clinker balls every so often to ensure that their chemical composition matches expectations.
Clinker is either sold as is or ground in situ with other materials, such as gypsum, to make various types of cement.

